Improving Patient Care Through Teletrials
Online Course
Providing Clinical trial related training to our workforce to increase the number of “trial ready” sites in Victoria.
This comprehensive training program aims to enhance the capacity of our workforce to facilitate the successful implementation of teletrials, thereby increasing the number of "trial ready" sites in Victoria.
The focus is on improving patient care by adopting the teletrial model, which offers a participant-centred approach and brings about transformative changes in clinical trial processes.
The "Improving Patient Care through Teletrials" training modules will provide participants with a deep understanding of the teletrial model and its differentiation from traditional clinical trial models. This includes a detailed explanation of the modifications required in the processes of feasibility, initiation, maintenance, and closeout of a study in the context of teletrials.
What will I learn?
Learning Outcome:
Upon completion of this training program, participants will be able to:
-
Explain the overarching concept of teletrials:
-
teletrials are participant-centred
-
deliver a greater engagement in research activity
-
build clinical trial capacity at satellite sites
-
build the capability of trial professionals
-
-
promote the benefits of using teletrials as a model of care.
What are the modules?
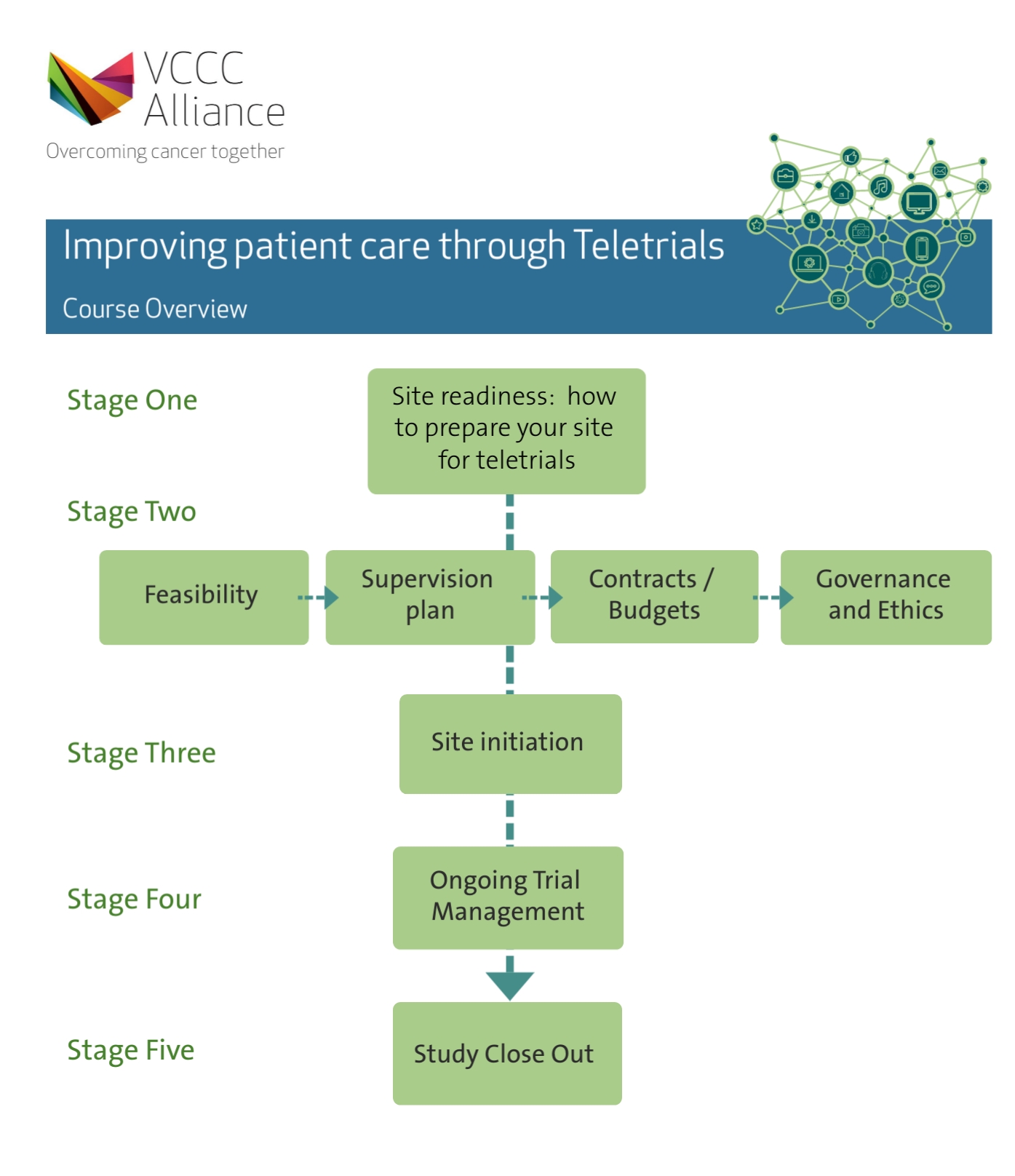
There are nine modules in the suite:
-
What is a teletrial?
-
Site Readiness
-
Feasibility and the Start-up Process
-
Budgets, Contracts & Indemnity
-
Ethics & Governance in Teletrials
-
Teletrials Site Initiation Visit
-
Study Close Out
-
Ongoing Teletrial Management
-
Teletrials Supervision Plan
This course equips participants with the knowledge and skills necessary to navigate teletrial implementation, ultimately contributing to the enhancement of patient care through innovative clinical trial approaches.
How to use these Modules
The purpose if these modules is to address the differences in practice that are required specifically for teletrials and are not intended to be a complete guide to the process of conducting a clinical trial in Australia.
For sites that have never conducted a clinical trial and require training from first principles, we would recommend completing a training course. Short courses in clinical trials are available for purchase through local universities and training providers.
Researchers conducting clinical trials are expected to have prior knowledge and an excellent understanding of the following documents:
The National Statement on Ethical Conduct in Human Research (2007) - Updated 2018
The National Statement on Ethical Conduct in Human Research (2007) - Updated 2018, sets national standards for use by any individual, institution or organisation conducting human research. This includes human research undertaken by governments, industry, private individuals, organisations, or networks of organisations.
The National Clinical Trials Governance Framework and User Guide
In February 2022, all jurisdictions agreed to implement the Governance Framework in health service organisations as an embedded approach (as clinical research is core health service business) under the Australian Health Service Safety and Quality Accreditation (AHSSQA) Scheme.
Australian Code for the Responsible Conduct of Research
All Australian sites are bound to abide by the Australian Code for the Responsible Conduct of Research. Adherence to the 2018 Code is a prerequisite for the receipt of funding by the National Health and Medical Research Council.
The Australian Clinical Trial Handbook
The Australian Clinical Trial Handbook provides guidance on the legislative, regulatory and good clinical practice (GCP) requirements when conducting clinical trials in Australia using 'unapproved' therapeutic goods. It assists trial sponsors, Human Research Ethics Committees (HRECs), investigators and approving authorities (institutions) to understand their roles and responsibilities under the therapeutic goods legislation.
https://www.tga.gov.au/resources/resource/guidance/australian-clinical-trial-handbook
Further Resources for Clinical Trials in Australia

Acknowledgements
The VCCC Alliance would like to acknowledge the following people for their contribution to this course:
Project Lead
Jessica Freeman, Regional Innovations Project Manager (VCCC Alliance)
Education Development and Design
The development of this toolkit was supported by Parkville Cancer Clinical Trials Unit (PCCTU).
William Evans,Teletrial Toolkit Development Coordinator (Parkville Cancer Clinical Trials Unit (PCCTU))
Amanda Clifford, Senior Research Nurse in Haem C Parkville Cancer Clinical Trials Unit
Alicia Mew, eLearning Developer (VCCC Alliance)
Teletrial Expert group:
Donna Long: Network Manager, Regional Trials Network - Victoria (RTN-Vic)
Narelle McPhee: Cancer Research Manager (Bendigo Cancer Centre)
Heidi Gaulke: Manager, Office for Research (Austin Health)
Ashlin Keane: Ethics Submission Specialist (Cancer Trials Australia)
Melanie Poxton: ADON Qld Regional Clinical trial Coordinating Centre (QRCCC)
Dr Kara Burns: Centre for Digital Transformation of Health (University of Melbourne)
William Evans: Teletrial Toolkit Development Coordinator (PCCTU, TrialHub Alfred Health)
Project Working Group
Chair: Associate Professor Kate Burbury (Peter MacCallum)
Narelle McPhee: Cancer Research Manager (Bendigo Cancer Centre)
Dr Dayna Swiatek: Manager (Victorian Cancer Agency)
Prof Mark Shackleton: Director of Oncology (Alfred Health)
Donna Long: Network Manager Regional Trials Network – Victoria (RTN-Vic)
Kathleen Wilkins: Consumer
Heidi Gaulke: Manager, Office for Research (Austin Health)
Sponsor Expert Group
Megan Foggin: IQVIA
Francis Hinds: Servier
Danielle Osmond: ANZGOG
Ryan Clarke: BMS
Lauren Bourke: IQVIA
Nicoline Wedemeyer: Roche
Stephanie Tan: Roche
Clinician expert group
Kate Burbury: Peter Mac
Nora Lee: Peter Mac
Javier Torres: Goulburn Valley Health
Amit Khot: Peter Mac
Sharad Sharma: Grampians Health
Other acknowledgement:
Lea-Anne Harrison: Regional Innovations Program Manager (VCCC Alliance)
Kaye McDowall: Regional Partnerships Implementation Manager (VCCC Alliance)
DJPR project funding has been utilised to support costs associated with development of resources including video production and online learning design of the VCCC Alliance teletrials training and education module, ”What is a teletrial and why should we do them?”
This teletrials toolkit education module and the implementation of resources will support the increase in uptake of teletrials through site capability.
Module one will be complemented by a suite of teletrials education and training resources on teletrials from site feasibility to study close out, as well as a teletrials toolkit developed in collaboration with national stakeholders.

Resource details
This course is brought to you by
