Investigator Initiated Trials (IITs) Online Course
This course aims to enable investigators to answer new clinical questions by creating Investigator Initiated Trials which in turn increases access to clinical trials for patients.
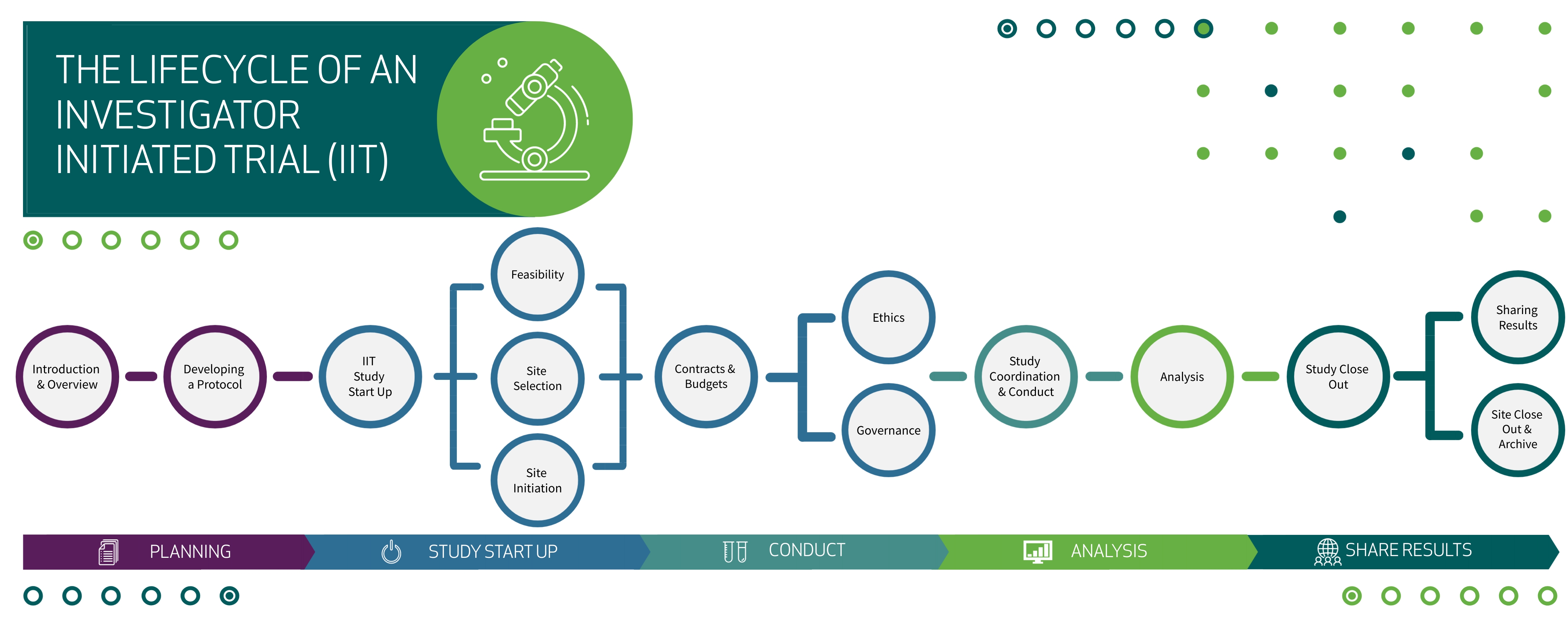
This course will follow the life of an IIT over 8 educational modules:
VCCC Alliance IIT Toolkit
The content of this course is based on the VCCC Alliance IIT resources for researchers.
Click here to access the toolkit.
 The toolkit was developed by the VCCC Alliance in partnership with the Melbourne Academic Centre for Health (MACH) and with assistance from the Melbourne Children's Trials Centre. It is comprised of a suite of resources to support researchers conducting investigator-initiated trials.
The toolkit was developed by the VCCC Alliance in partnership with the Melbourne Academic Centre for Health (MACH) and with assistance from the Melbourne Children's Trials Centre. It is comprised of a suite of resources to support researchers conducting investigator-initiated trials.
These resources have now been adapted into this interactive educational course.
Course Topics
Planning: Introduction to IITs & Developing a Protocol
Study Start Up: Feasibility, Contracts & Budget, & Ethics & Governance
Study Conduct: Study Coordination and Ongoing Management
Data Analysis: Biostatistical Analysis at every stage of a trial
Sharing Results: Study Close Out, Results dissemination, Study Archiving
Below is a visual overview of the Investigator Initiated Trial (IIT) life cycle. This course will follow the life of an IIT over 8 educational modules.
Aim
This course aims to enable investigators to answer new clinical questions by creating Investigator Initiated Trials which in turn increases access to clinical trials for patients.
Learning Objectives
During this course, you will have the opportunity to:
Explore the lifecycle stages in an Investigator-Initiated Trial and the considerations for early phase Investigator-Initiated Trials
Explore structured guidance in developing an Investigator Initiated Trial protocol.
Explore essential groundwork to initiate a clinical trial, emphasising a robust feasibility assessment process through to site initiation.
Explore contracts and common agreements that are used in the management of Investigator Initiated Trials (IITs) in Australia and considerations for budgeting for IITs.
Describe the preparation for ethics and governance review, the submission process, and the ongoing responsibilities of the Sponsoring Institution and participating trial sites after approval.
Describe key activities, tasks, and responsibilities during the conduct of an Investigator Initiated Trial.
Outline the key elements of trial data analysis including the involvement of a Biostatistician in all stages of an IIT.
Describe the process of sharing IIT result data with the scientific community, external collaborators, and participants
Learning Outcome
By the end of the course, you will have the fundamental knowledge to follow the lifecycle of an IIT and identify key considerations when in the planning phase of an IIT.
Note: These resources are supported with advisory guidance and links to relevant guidelines to provide researchers with a launching pad for conducting their research.
Course Contributors
The VCCC Alliance would like to thank the following people for their expert contribution to the course:
Dr Kortnye Smith, Medical Oncologist and Researcher, Peter MacCallum Cancer Centre and Eastern Health
Amanda Clifford, Clinical Trials Research Nurse, Peter MacCallum Cancer Centre
Duncan Colyer, Senior Manager Clinical Research, VCCC Alliance
Project Manager
Elizabeth Diao, Project Officer - Accelerating Novel Therapies, VCCC Alliance
VCCC Alliance in partnership
The toolkit was developed by the VCCC Alliance in partnership with the Melbourne Academic Centre for Health (MACH) and with assistance from the Melbourne Children's Trials Centre. It is comprised of a suite of resources to support researchers conducting investigator-initiated trials.

These resources have now been adapted into this interactive educational course.
Resource details

This course is brought to you by
