Increasing Awareness and Access to Early-Phase Clinical Trials
Aim: The VCCC Alliance's project on Increasing Awareness & Access to Early-Phase Clinical Trials aims to provide accessible information about early-phase clinical trials for both consumers and clinical trial staff, including research coordinators and clinicians.
Learning Objectives:
-
Develop recommendations for consumer-facing early phase clinical trial information
-
Provide equitable consumer-facing early phase clinical trial information
Developing Recommendations for Consumer-facing Early Phase Clinical Trial Information
Recruiting participants for clinical trials poses a considerable challenge, particularly for early-phase clinical trials, which present unique challenges due to their specialised nature and the timing of their offerings.
Providing adequate information to support a consumer's decision to participate is complex, compounded by prevailing myths that label participants as "guinea pigs." In response, the VCCC Alliance's Accelerating Novel Therapies program collaborated with member sites (Western Health, Peter MacCallum Cancer Centre, Austin Health, and St. Vincent's) to conduct a quality improvement exercise and produce materials that cater to consumer needs. A literature review and scoping exercise, including collaboration with Clinical Trials Units (CTUs) internationally, were conducted to understand the provision of information on early-phase clinical trials. Focus groups, involving consumers and advocates, reviewed available materials and communication methods, leading to 'best practice' recommendations.
Recommendations
Recommendation for Early Phase Clinical Trial Information Providers
- Include the entire clinical trials team, with specialists, clinical trials team members, and GPs recognized as trustworthy sources.
- Allocate sufficient time for discussion and information processing, involving resources and staff for post-provision discussions with GPs, specialists, trials staff, family, and friends.
- Expand discussion scope to include wider consumer concerns such as palliative care, logistics, financial costs, and discussions around hope.
Recommendation on What to Include in Early Phase Clinical Trial Information
- Direct consumers to trusted resources using recommendations from reliable sources.
- Provide clear, staged information to cater to varying health literacy levels
- Early phase clinical trial patients were more likely to have additional considerations impacting their ability to receive education, such as receiving large volumes of information from multiple sources and specialists, or “chemo fog” - information needs to be clear
- Consider different formats, including hard copies alongside digital formats
- A range of resources allowed for differing needs – e.g., general clinical trial information, specific trial information and question prompt tools.
- Ensure cultural relevance and accessibility, including translations.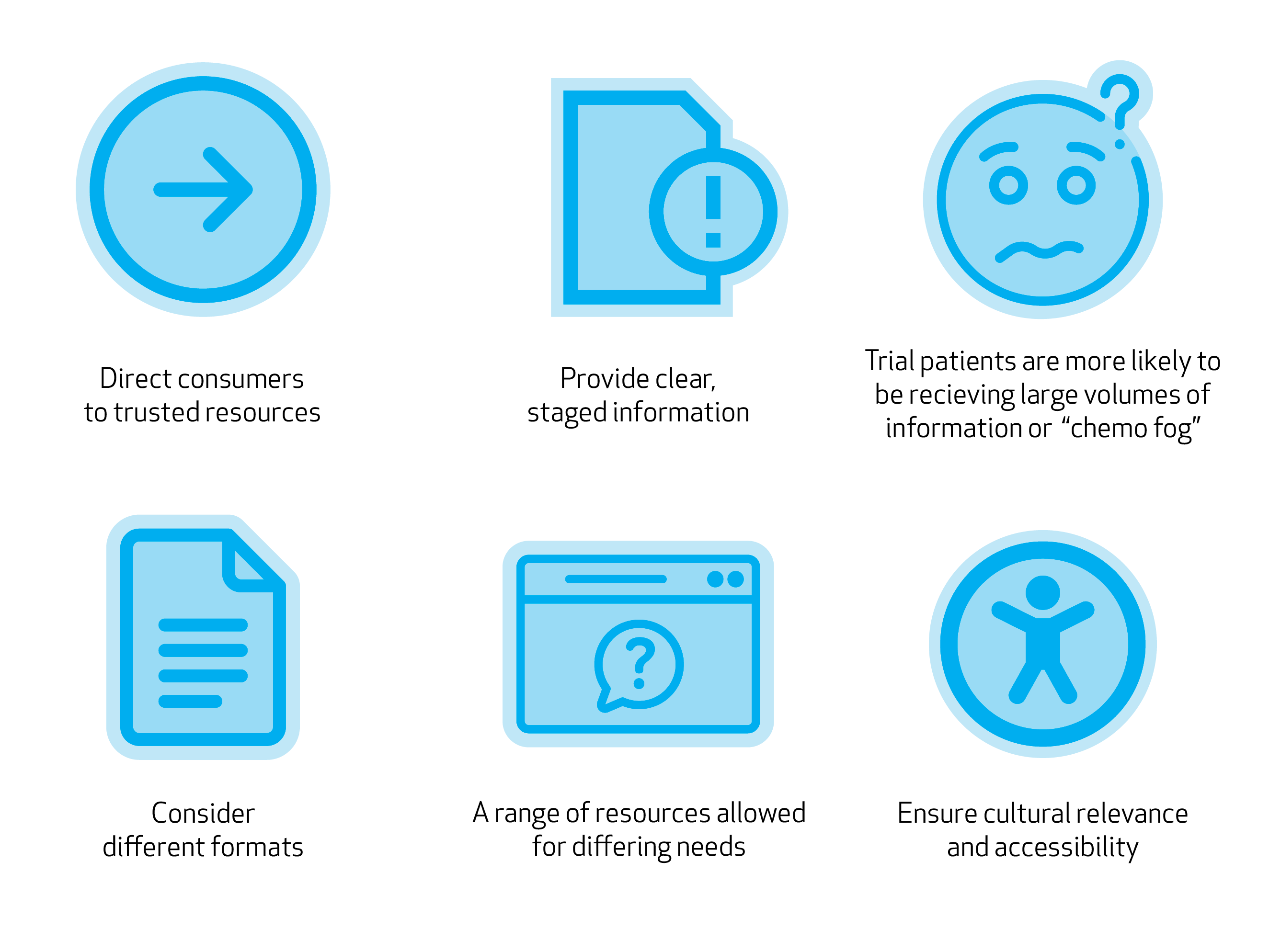
Equitable Consumer-facing Early Phase Clinical Trial Information
Following comprehensive scoping and review, the project team sought examples of clinical trial information aligning with the identified recommendations. In collaboration with the Parkville Cancer Clinical Trials Unit (PCCTU), the VCCC Alliance Accelerating Novel Therapies team translated "Is a Clinical Trial Right for You?" into 17 languages.
Find out more
If you have questions about the Increasing Awareness & Access to Early Phase Clinical Trials project, or would like to know more about this project, contact:
Elizabeth Diao, Project Officer VCCC Alliance
elizabeth.diao@vcccalliance.org.au
Duncan Colyer, Senior Manager, Clinical Research, VCCC Alliance
duncan.colyer@vcccalliance.org.au
Resource details

This course is brought to you by
